Posted: October 27th, 2016 | Author: Shauna | Filed under: Basics, Controlled Substances, In the News, Public Policy, Security, Standards | Tags: Controlled Substances, DEA, DoseSpot, e-Prescribing, e-Prescribing controlled substances, e-Prescribing Integration, e-Prescribing Software, electronic prescribing, Healthcare Software, Opioid Epidemic, Opioids, Patient Engagement, PDMP, PMP, Prescription Drug Monitoring Program, Prescription Monitoring Program, State Mandates, trends, Value Based Care | No Comments »
On par with our last post, the widespread media attention and devastating losses associated with our nation’s current opioid epidemic has sparked certain state legislatures to regulate and improve providers’ prescribing habits for prescription painkillers.
With good intentions in tow, some rulings seem to lack readily available solutions that are proven to curb this crisis. However, they do realize that their recent proposals do not mark the end of this uphill battle, rather multifaceted solutions need to be in place to truly, and successfully, overcome this epidemic.
[Read: Overdose Awareness – The Time to Stand Together is Now]
Here are three states that have recently proposed rulings on how opioids should be prescribed:
Vermont
Coined as a “cutting-edge†approach to overcoming the opioid crisis, Governor Peter Schumlin announced proposed limits on the number of opioid medications that could be prescribed.
Like every other state, Vermont has seen an incredible increase in deaths related to opioid and heroin overdose in recent years and Governor Schumlin is no longer sitting on the sidelines.
Earlier this year, he approached both the FDA and pharmaceutical industry in his State of the State address claiming that OxyContin “lit the match that ignited America’s opiate and heroin addiction crisis,†and that the booming American opiate industry knows no shame, an outcry after the FDA approved OxyContin for children a few months ago.
The proposed ruling states that the severity and duration of pain will determine the specific limit for a prescription of opioids. For example, a minor procedure with moderate pain would be limited to 9-12 opioid pills and the amount would increase based on the procedure performed and the level of pain a patient claims. The ruling would also require providers to discuss risks, provide an education sheet to the patient and receive an informed consent for all first-time opioid prescriptions.
The Green Mountain State’s Governor believes that limiting the number of opioid pills prescribed would be an effective way to reduce addiction, yet some folks believe the ruling would only encourage patients to seek illicit drugs elsewhere if they cannot receive pain medication through their provider.
This does make sense considering many former and current heroin abusers have stated that their addiction started from a prescription and when the pill bottle ran out, they were left seeking these drugs on the streets, which have proven to be very, if not more, dangerous than the prescription.
However, the intent of the Governor’s ruling is to prevent addiction from ever happening in the first place. His ruling is specific to cases of acute pain, therefore changing the over-prescribing habits and learned behavior of utilizing opioids as first-line therapy; habits that ensued in large part due to incentives, the surge of pharmaceutical marketing tactics and claims that painkillers were not addictive.
[Read: How Costly Are Prescription Pain Meds?]
New Jersey
With the rate of drug overdose deaths on the rise by 137% since 2000, New Jersey is another state to recently propose new regulations on how and to whom opioids are prescribed.
New Jersey, much like many other states, believes that prevention is key when fighting this crisis and they couldn’t be more correct. Unfortunately, several barriers often occur when seeking appropriate treatment after a patient becomes addicted, (for example, providers are limited to certain amounts for which they can administer reversal drugs), and therefore why not PREVENT addiction, rather than simply TREAT addiction when at many times, it’s too late?
Senator Raymond Lesniak has introduced a bill that would put restrictions on health insurance coverage for opioid medications, while also requiring prescribers to first consider alternative pain-management treatments, follow federal prescribing guidelines and explain the risk of addiction with such substances to their patients before prescribing. Furthermore, providers will need to complete several steps before receiving approval of an opioid prescription. These steps include providing a patient’s medical history, conducting a physical exam and developing an appropriate medical plan for treating a patient’s pain.
While new rulings in place can certainly shift this epidemic, Angela Valente, the executive director of the Partnership for a Drug-Free New Jersey, said it best:
“Awareness and education is the key factor in preventing the abuse of opiates—everyone must have a role in reversing this epidemic, including lawmakers, parents, coaches, educators, and yes, even doctors and dentists.†– Angela Valente
Dr. Andrew Kolodny, executive director of Physicians Responsible for Opioid Prescribing, further backs Valente’s point while also motioning that the medical community has been prescribing too aggressively.
[Read: The Opioid Epidemic: Are Dentists the Black Sheep?]
Pennsylvania
Unfortunately, Pennsylvania experienced 3,500 deaths last year as a result from drug overdose, one of the highest overdose rates in the nation.
The state has had a Prescription Drug Monitoring Program for quite a few years now, however it wasn’t functional until August 2016, when their new program was officially rolled out. Pennsylvania requires providers to query the state’s prescription drug database the first time they prescribe a controlled substance to a patient or if they have reason to believe that the patient is doctor shopping.
Governor Tom Wolf addressed other initiatives underway including requiring providers to query the database EACH time they prescribe opioids, updating medical school curriculum and continuing education, changes to the process of pain care to lower inappropriate use of opioids, and improved screening, referral and treatment for addiction.
What’s bothersome in Pennsylvania, is the method in which these substances have to be prescribed. The Pennsylvania Controlled Substance Act requires narcotic prescriptions to be handwritten on paper prescription pads, yet every other substance can be electronically prescribed. This allows the risk of written prescriptions being lost, stolen, or sold. Luckily, Senator Richard Alloway intends to introduce this measure before the legislative session’s end.
It’s promising to see how the above states are utilizing their state’s Prescription Drug Monitoring Program, or PDMP. All three require their prescribers to query the affiliated state database, however the parameters in which, or how often, they check varies.
While said efforts are better than no effort at all and states are starting to fully understand the need for multifaceted solutions in order to overcome this epidemic, one key solution is missing. E-Prescribing.
[Read: The Link Between PDMP’s and e-Prescribing]
How does e-Prescribing help combat this epidemic?
- e-Prescribing diminishes the possibilities of duplicate or lost prescriptions since the prescription is sent directly to the patient’s pharmacy
- A patient will no longer have a paper prescription where the dispense quantity can be altered
- Prescribers will have access to a patient’s medication history, therefore they can determine if a patient is “doctor shopping†or has a history of substance abuse
To learn how to incorporate e-Prescribing as a solution to the opioid epidemic, schedule a meeting with DoseSpot today.
Sources:Â NY Times; Boston.com; ABC News; Press of Atlantic City; PennLive
About DoseSpot
DoseSpot is a Surescriptsâ„¢ certified e-Prescribing platform specifically designed to integrate with electronic health record, electronic dental record, practice management and telehealth software. DoseSpot is certified to e-Prescribe controlled substances and has provided simple, affordable and integratable e-Prescribing solutions to healthcare IT companies since 2009. For more information, please visit www.DoseSpot.com.
Posted: October 20th, 2016 | Author: Shauna | Filed under: Controlled Substances, In the News, Standards | Tags: Controlled Substances, DEA, e-Prescribing, e-Prescribing controlled substances, MA PAT, MA PMP, Massachusetts PMP, Massachusetts Prescription Awareness Tool, Massachusetts Prescription Monitoring Program, Massachusetts State Mandate, MassPAT, Opioid Epidemic, Opioids, Patient Engagement, PDMP, PMP, Prescription Drug Monitoring Program, Prescription Monitoring Program, State Mandates, Value Based Care | No Comments »
For the first 6 months of 2016 in Massachusetts, there have been almost 500 confirmed cases of unintentional opioid overdose deaths and an estimated 500 additional cases have not yet been confirmed.
The majority of overdoses found in MA are due to substances such as fentanyl and heroin, but rates of cocaine and benzodiazepines present in opioid deaths have been steady since 2014. Although the rates of heroin and prescription drugs present in opioid deaths have been decreasing due to many efforts that have been implemented across the nation, the rate of fentanyl has been on the rise. This is in large part due to the fact that many opioid addictions start at the hands of a prescriber with a prescription and when the pill bottle runs dry, patients are left seeking other options that produce the same euphoric effect.

With the rapid increase of deaths and devastation by way of the current opioid epidemic plaguing the state, Massachusetts has recently implemented further requirements concerning practitioner’s prescribing protocols. Specifically, with the state’s Prescription Monitoring Program, or PMP.
The PMP serves as a database for all prescription drugs that are dispensed across the state, including those that are highly sought after for non-medical use and represent the highest potential for abuse, better known as Schedule II-V drugs such as narcotics, sedatives, and stimulants.
When properly used, the PMP aids in the identification and prevention of drug misuse, diversion, and potential doctor shopping by providing a patient’s medication history of the past 12 months. It is meant to be utilized as a key clinical decision-making tool that allows providers to receive a big picture view of the patient they are treating in real time.
As a solution to this widespread epidemic, Massachusetts has introduced new legislation and requirements when utilizing the MassPAT (Massachusetts Prescription Awareness Tool).
Effective October 15, 2016, practitioners must abide by the following:
- A registered individual practitioner must utilize the prescription monitoring program each time the practitioner issues a prescription to a patient EACH time for a narcotic drug in Schedule II or III.
- A registered individual practitioner must utilize the prescription monitoring program prior to prescribing to a patient for the first time:
- A benzodiazepine; OR
- Any controlled substance in Scheduled IV or V which the department has designated in guidance as a drug that is commonly abused and may lead to dependence. At this time, there are no drugs that have received this designation.
Prior to the aforementioned requirements, legislation ruled that practitioners, among other factors, need only check the state PMP when prescribing a controlled substance to a patient for the first time, while it is now required for a practitioner to check the system EVERY time when prescribing Schedule II or III drugs.
An example of just how serious Massachusetts is about this crisis, and also believed to be the first agreement of its kind, CVS recently paid almost $800k to the state because pharmacists were not checking prescriptions or the database thoroughly. In exchange, CVS agreed to provide its pharmacists access to the PMP website, train its pharmacists to register for and use the PMP as appropriate, and has further agreed to implement policies that would require pharmacists to consult the PMP before dispensing certain opioids in MA.
Massachusetts and CVS, among many other organizations, recognize the importance of the state’s PMP as a tool to detect and prevent the abuse and misuse of controlled substances. The PMP is not meant to be another government-controlled, green monster hanging on a practitioner’s back at all times; it is meant to serve as a safety extension for practitioners, but most importantly for their patients.
PMP’s can also be most effective when linked with an e-Prescribing solution. Working together, e-Prescribing eliminates the need for paper prescriptions, thus reducing the risk of altered dispense quantities, stolen prescriptions or prescription pads, and the reselling of such prescriptions before they’re filled as a means of lessening the red flags if a patient is doctor shopping.
About DoseSpot
DoseSpot is a Surescriptsâ„¢ certified e-Prescribing platform specifically designed to integrate with electronic health record, electronic dental record, practice management and telehealth software. DoseSpot is certified to e-Prescribe controlled substances and has provided simple, affordable and integratable e-Prescribing solutions to healthcare IT companies since 2009. For more information, please visit www.DoseSpot.com.
Posted: September 8th, 2016 | Author: Shauna | Filed under: Basics, In the News, Standards | Tags: Baby Doctor, Company Spotlight, health IT, Healthcare Startups, Innovative, On-Demand Healthcare, Patient Engagement, Pediatrics, Personalized Medicine, Startup, Value Based Care | No Comments »
Coined as “The Uber of Pediatricians,” DoseSpot customer, Baby Doctor is making waves with their latest on-demand services connecting parents with doctors.
Does your child have a sinus infection? A stomachache? How about a sore throat? Baby Doctor has you covered. Happy, healthy kids is their company’s mission and by implementing a service of boutique-style, personalized house calls, they are able to provide just that.
By using their website or app, parents are able to request a visit from a Pediatrician, Pediatric Nurse Practitioner, or Family Nurse Practitioner for a variety of symptoms a child may be experiencing and the provider appears at their home within 60 minutes or less.
This not only benefits parents, but insurance providers and emergency rooms throughout the city as well since it collectively saves them all time and money.
An average ER visit can cost upwards of $1,500 and two-thirds of ER visits aren’t true emergencies, but Baby Doctor offers their on-demand services for a fraction of the cost. Though they’re not considered an in-network provider, at least not yet anyways, Baby Doctor offers an itemized receipt for parents to submit to their insurance company for out-of-network reimbursement.
With their 50 board certified providers in Manhattan, Baby Doctor is hoping to expand to Brooklyn, the Bronx, and Queens by end of 2016, with further expansion into New Jersey come 2017.
By paving the way with their unique delivery model, Baby Doctor is devoted to providing an efficient, personalized and affordable means of healthcare to parents for their children, all while broadening patient engagement and levels of satisfaction.
Stay tuned for this blossoming company as it will undoubtedly remain at the forefront of on-demand innovation.
For more information on Baby Doctor, please visit their website: www.BabyDoctor.com.
About DoseSpot
DoseSpot is a Surescripts certified e-Prescribing platform specifically designed to integrate with electronic health record, electronic dental record, practice management and telehealth software. DoseSpot is certified to e-Prescribe controlled substances and has provided simple, affordable and integratable e-Prescribing solutions to healthcare IT companies since 2009. For more information, please visit www.DoseSpot.com.
Posted: July 1st, 2015 | Author: Lindsay | Filed under: Basics, Controlled Substances, In the News, Security, Standards | Tags: Controlled Substances, DEA, DoseSpot, DoseSpot integration, e-Prescribing, EPCS, Healthcare Software, medication adherence, medication safety, medication tracking, Patient Engagement, prescription monitoring | No Comments »
Over the past few years, prescription drug abuse has been a heated topic here in the U.S. among healthcare professionals and policymakers alike. Engineering students within Johns Hopkins University’s Whiting School of Engineering are taking strides to address continuously alarming drug abuse statistics with the creation of a novel, tamper-proof pill bottle.
As cited in HIStalk Connect’s article, the engineering students were called upon by the Johns Hopkins Bloomberg School of Public Health to undertake this special project. With more than 16,000 annual deaths attributed to prescription drug-related overdoses, the goal of Hopkins’ project was to develop a robust pill bottle that would help control the nation’s relatively unsecured supply of prescription narcotics. According to assistant professor at the Bloomberg School of Public Health, Kavi Bhalla, the overseeing team wanted “this personal pill ‘safe’ to have tamper resistance, personal identification capabilities and a locking mechanism that allows only a pharmacist to load the device with pills.”
The four engineering undergrads assigned to take on this project answered accordingly–by developing a 2.75 pound, nine-inch-tall, steel-constructed pill bottle that can withstand any hammer or drill activity. Additionally, fingerprint scanners are used to regulate dispensing and ensure that pills are only released to the patient a medication is prescribed to–at proper time intervals and in correct doses. After gaining positive feedback from both Bloomberg clinicians and pharmacists at the on-campus Rite Aid, Hopkins engineering students uncovered an important and overlooked design value: the ability to record medication adherence rates. If connected to a monitoring system, the tamper-proof pill bottle (again, equipped with fingerprint reading capabilities) could eventually be useful to payers and health systems working to reduce funds wasted on poor medication adherence.
For healthcare software companies looking to incorporate the ability to electronically prescribe controlled substances (EPCS), DoseSpot could be your solution of choice in just a few hours, days or weeks! Through our third party EPCS audit with Drummond Group Inc., a global software test and certification body that is approved by the Drug Enforcement Administration to audit EPCS software applications, DoseSpot is now able to deliver audited and trusted EPCS software applications to customers. For more information on DoseSpot’s EPCS software, please download our Integration Tool Kit here!
SOURCE: HIStalk
About DoseSpotÂ
DoseSpot is a Surescripts certified e-Prescribing platform specifically designed to integrate with electronic health record, electronic dental record, practice management and telehealth software. DoseSpot is certified to e-Prescribe controlled substances and has provided simple, affordable and integratable e-Prescribing solutions to healthcare IT companies since 2009. For more information, please visit http://www.DoseSpot.com.
Posted: April 17th, 2015 | Author: Lindsay | Filed under: Basics, Controlled Substances, In the News, Standards | Tags: consumer apps, Controlled Substances, digital health, DoseSpot, e-Prescribing, EPCS, Healthcare Software, integration, iOS, NJPMP, prescription monitoring | No Comments »
Earlier this week, New Jersey’s Division of Consumer Affairs announced the launch of a new iPhone app that will allow authorized users of the state’s prescription drug monitoring program—namely pharmacists and prescribers licensed in New Jersey—to access the crucial database via their smartphone or tablet. The New Jersey Prescription Drug Monitoring Program (NJPMP) operates under the Division of Consumer Affairs and this state department is also responsible for managing and updating the prescription drug database.
The NJPMP collects data on prescriptions for controlled substances filled in the state of New Jersey, including opiate painkillers and a variety of narcotics. Prescribers can conveniently use the database to locate patients who may be “doctor shopping,†which the NJPMP defines as “deceptively visiting multiple physicians to obtain more prescription drugs than [a single doctor] would prescribe†or “trying to illegally obtain prescription drugs through use of multiple pharmacies.â€
Officially launching in the year 2011, the NJPMP views this new iPhone app as the latest in a recurring series of upgrades that the Program has laid out for the coming months. A major goal, according to acting Attorney general John J. Hoffman, is to make the NJPMP as user-friendly as possible—thus increasing adoption rates among prescribers and pharmacists, whose participation in actively addressing prescription drug abuse is critical.
According to the NJPMP, 88.4% of the state’s 29,400 licensed prescribers are registered to use the NJPMP and between March and April of 2015, 169,000 user requests were submitted. The app is currently only available for iPhones and iPads, however, the NJPMP plans to launch both Android and Windows apps by the summer.
For more information on the New Jersey Prescription Drug Monitoring Program, check out a brief released by the Department of Consumer Affairs here!
SOURCE: mobihealthnewsÂ
About DoseSpotÂ
DoseSpot is a Surescripts certified e-Prescribing platform specifically designed to integrate with electronic health record, electronic dental record, practice management and telehealth software. DoseSpot is certified to e-Prescribe controlled substances and has provided simple, affordable and integratable e-Prescribing solutions to healthcare IT companies since 2009. For more information, please visit www.DoseSpot.com.
Posted: February 19th, 2015 | Author: Jodi | Filed under: Basics, Controlled Substances, Dental, In the News, Standards | Tags: Dental, DoseSpot, e-Prescribing, EPCS, healthIT, technology, trends | No Comments »
Needham Heights, MA (PRWEB) February 19, 2015
DoseSpot, an industry leader in e-Prescribing integration platforms for medical, dental and telehealth software, today announced that Professional Economics Bureau of America’s XLDent has successfully integrated DoseSpot’s EPCS certified module for Electronic Prescribing of Controlled Substances (EPCS). In addition, XLDent successfully completed an EPCS integration review by Drummond Group and XLDent’s dental customers are now equipped to e-Prescribe controlled substances in 49 states.
“Following DoseSpot’s successful completion of EPCS certification last year, we are thrilled to be working with our existing customer base to provide EPCS capabilities within our easy-to-use prescribing interface†said Greg Waldstreicher, CEO, DoseSpot. The certification comes in advance of the New York state mandate whereby all prescriptions must be sent electronically including controlled substances. The mandate goes into effect March 27, 2015 and will have a major impact on the dental industry as dental providers are most frequently prescribing a combination of antibiotics and controlled substances.
The DoseSpot Dental platform integrated with XLDent offers dentists the ability to route e-Prescriptions to the patient’s pharmacy of choice after automatically checking for any drug-drug and drug-allergy interactions. “With the NY mandate on the horizon and recent reclassification of hydrocodone combination medications, it’s critical for dental providers to adopt an e-Prescribing solution capable of sending all prescriptions electronically†added Greg. “XLDent continues to be at the forefront of dental e-Prescribing and we’re excited to see what the future has in store for e-Prescribing in dentistry.â€
“XLDent partnered with DoseSpot in 2011 to offer integrated e-Prescribing services when it was mandated by the state of Minnesota and now we’re excited to be one of the first dental software solutions to integrate EPCS capabilities into our Dental Practice Management and Electronic Dental Record offering†said Dawn Christodoulou, President, Professional Economics Bureau of America, Inc. “It continues to be a pleasure to work with DoseSpot, a company that shares in our vision to bring valuable, patient-centered solutions to the table.â€
For more information on DoseSpot’s EPCS software, please visit http://www.DoseSpot.com or contact Lindsay Walsh, Lindsay(at)dosespot(dot)com.
About XLDentÂ
The XLDent dental practice management system takes advantage of the latest in software development and design tools to provide its clients with the most intuitive, robust, easy-to-use dental program available. XLDent is an electronic dental records system that automates key business processes and ensures dental teams have the tools they need to manage the practice more profitably. Key benefits include a comprehensive suite of products written for a mobile, wireless, tablet PC environment. Other key benefits include seamlessly integrated e-Prescribing through DoseSpot, flexible Progress Note system, efficient clinical workflows, and dentist and Patient Portals. XLDent supports general and specialty dental clinics and is used by single practitioners as well as large multi-dentist, multi-location clinics nationwide.
About DoseSpotÂ
DoseSpot is a Surescripts certified e-Prescribing platform specifically designed to integrate with electronic health record, electronic dental record, practice management and telehealth software. DoseSpot is certified to e-Prescribe controlled substances and has provided simple, affordable and integratable e-Prescribing solutions to healthcare IT companies since 2009. For more information, please visit http://www.DoseSpot.com.
Read the full press release here:Â http://www.prweb.com/releases/2015/02/prweb12528541.htm
Posted: October 15th, 2014 | Author: Jodi | Filed under: Controlled Substances, In the News, Newsletter, Public Policy, Security, Standards | Tags: DEA, DoseSpot, Drummond Group, e-Prescribing Software, EPCS, healthIT, trends | No Comments »
Needham Heights, MA (PRWEB) October 15, 2014 - DoseSpot, an industry leader in e-Prescribing integration platforms for medical, dental and telehealth software, today announced that its software application has completed the required third-party Electronic Prescribing of Controlled Substances (EPCS) audit with Drummond Group Inc., a global software test and certification body that was approved by the Drug Enforcement Administration (DEA) to audit EPCS software applications.
DoseSpot selectively pursued EPCS Certification with Drummond Group, one of the first DEA approved certification bodies. “After undergoing Drummond Group’s extensive audit process and phased approach to understanding the EPCS requirements, DoseSpot may now deliver audited and trusted EPCS software to their customers,†said Aaron Gomez, Drummond Group’s Director of EPCS Auditing.
The audited EPCS software also incorporates industry leading two-factor authentication and identity proofing technologies to meet the requirements of the DEA Interim Final Rule for EPCS. “We strive to provide an easy-to-use e-Prescribing interface and our team has successfully incorporated the EPCS functionality without disrupting our existing user experience,†said Greg Waldstreicher, President, DoseSpot.
DoseSpot prescribers will now have the ability to e-Prescribe controlled substances in 49 states. “In less than six months, New York will be the first state to mandate e-Prescribing, including EPCS,†added Greg Waldstreicher. “We are committed to offering the best e-Prescribing integration experience for our current and future software customers and have made the process for enabling EPCS incredibly easy.â€
For more information on DoseSpot’s EPCS software, please visit http://www.DoseSpot.com or contact Lindsay Walsh, Lindsay(at)dosespot(dot)com.
About DoseSpotÂ
DoseSpot is a Surescripts™ certified e-Prescribing platform specifically designed to integrate with electronic health record, electronic dental record, practice management and telehealth software. DoseSpot has provided simple, affordable and integratable e-Prescribing solutions to healthcare IT companies since 2009. To request a demo of DoseSpot’s e-Prescribing integration platforms, please visit http://www.DoseSpot.com/.
About Drummond Group Inc.Â
Drummond Group Inc. is a global software test and certification lab and third-party auditor that serves a wide range of vertical industries. In healthcare, Drummond Group tests and certifies Controlled Substance Ordering Systems (CSOS), Electronic Prescription of Controlled Substances (EPCS) software and processes, and Electronic Health Records (EHRs) – designating the trusted test lab as the only third-party certifier/auditor of all three initiatives designed to move the industry toward a digital future. Founded in 1999, and accredited for the Office of the National Coordinator HIT Certification Program as an Authorized Certification Body (ACB) and an Authorized Test Lab (ATL), Drummond Group continues to build upon its deep experience and expertise necessary to deliver reliable and cost-effective services. For more information, please visit http://www.drummondgroup.com or email DGI(at)drummondgroup(dot)com.
Read the full press release here:Â http://www.prweb.com/releases/2014/10/prweb12249755.htm
Posted: July 2nd, 2014 | Author: Greg | Filed under: Basics, In the News, Standards, Telehealth, Venture funding | Tags: digital health, DoseSpot, e-Prescribing, e-Prescribing Integration, EHR, EHR software, Health Information Exchange, healthIT, meaningful use, rockhealth, surescripts, surescripts certification, trends, venture funding | No Comments »
RockHealth recently published their Digital Health Funding – Midyear Review and I’ve highlighted some key findings below:
In 2013 digital health companies raised $2 billion in venture funding…first six months of 2014 digital health companies have already raised $2.3 billion.
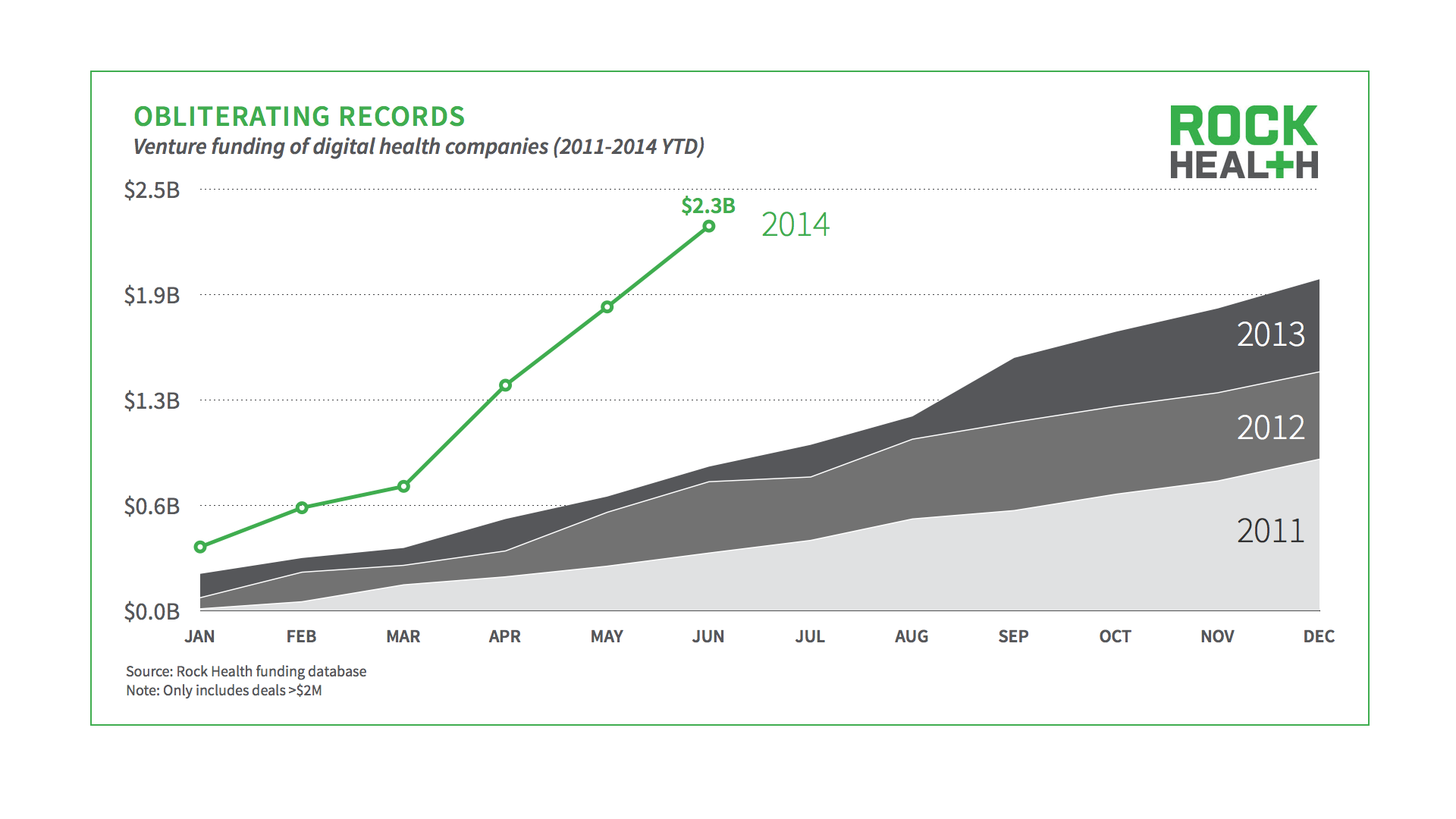
So where’s all the money going?
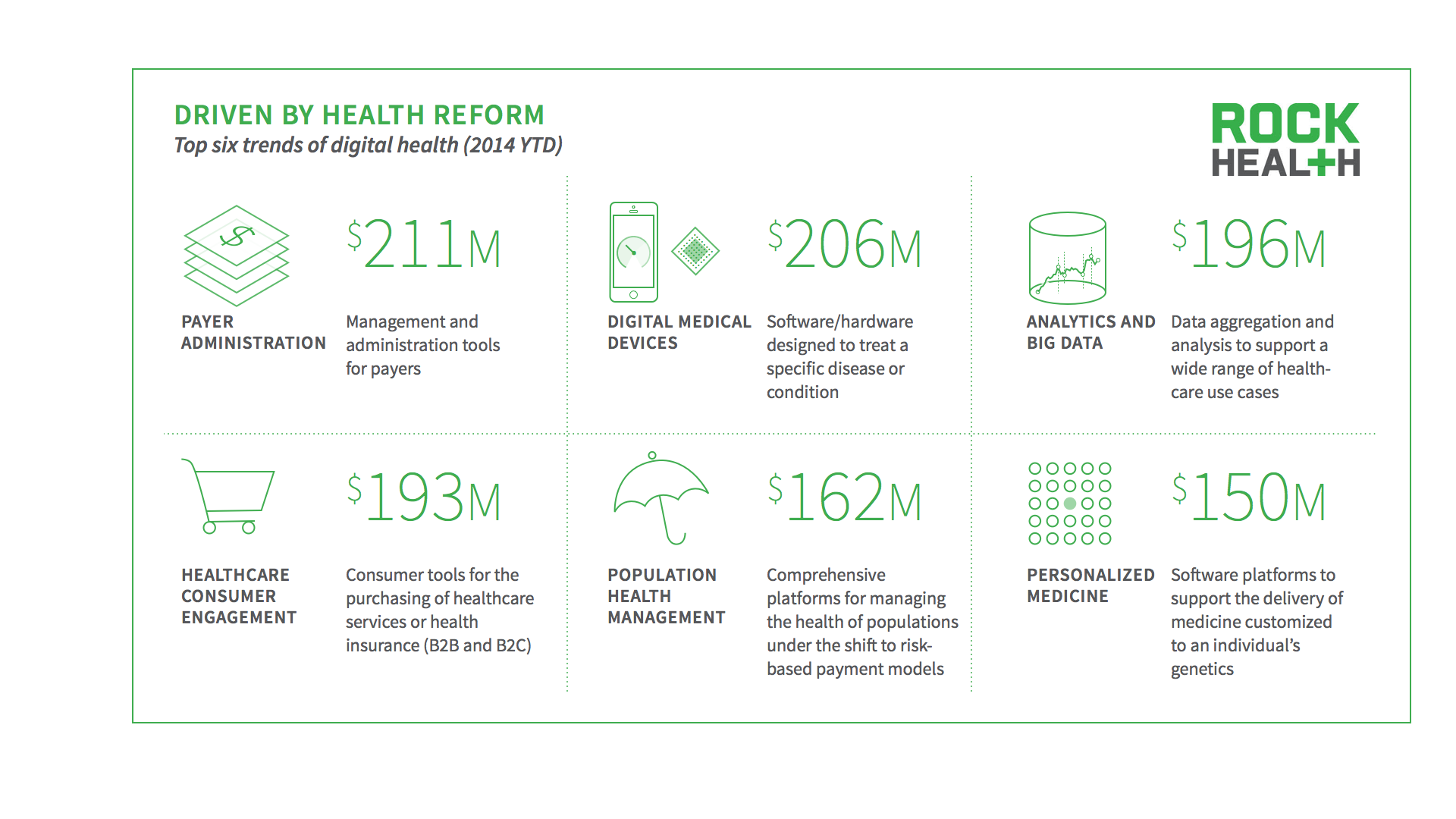
Here is the full report:
About DoseSpot
DoseSpot is a Surescripts™ certified e-Prescribing platform specifically designed to integrate with electronic health record, electronic dental record, practice management and telehealth software. DoseSpot has provided simple, affordable and integratable e-Prescribing solutions to healthcare IT companies since 2009. For more information, please visit www.DoseSpot.com.
Posted: June 25th, 2014 | Author: Greg | Filed under: Basics, In the News, Newsletter, Public Policy, Standards | Tags: #dosespotted, DoseSpot, e-Prescribing, e-Prescribing Integration, e-Prescribing Software, EHR, EHR software, healthIT, meaningful use, surescripts, surescripts certification | No Comments »
Surescripts recently published their annual National Progress Report highlighting advances in e-Prescribing throughout the United States. Without a doubt e-Prescribing is ‘here to stay’ and DoseSpot continues to be at the forefront of e-Prescribing integration services.
Below are a few key figures from the National Progress Report:
#1. More than 1.04 Billion e-Prescriptions were sent in 2013.

#2. 699 Million medication histories were delivered which often assist in limiting drug-drug interaction errors.

#3. Office-based physician adoption continues to grow and currently stands at 73%.

#4. 95% of pharmacies have adopted e-Prescribing.

About DoseSpot
DoseSpot is a Surescripts™ certified e-Prescribing platform specifically designed to integrate with electronic health record, electronic dental record, practice management and telehealth software. DoseSpot has provided simple, affordable and integratable e-Prescribing solutions to healthcare IT companies since 2009. For more information, please visit www.DoseSpot.com.
Posted: April 29th, 2014 | Author: Jodi | Filed under: Basics, In the News, Incentives, Newsletter, Public Policy, Standards | Tags: CMS, DoseSpot, e-Prescribing, e-Prescribing Software, EHR, EHR software, healthIT, meaningful use, MU, ONC, Stage 2 | No Comments »
Looking for tips to navigate MU stage 2 requirements?  View one tip on drug-drug and drug-allergy interaction checks below and visit http://www.dosespot.com/meaningful-use-tips to download all 5 certification tips!
Meaningful Use Stage 2:Â Tip #2Â –
§ 170.314(a)(2) Drug-drug, drug-allergy interaction checks
Objective:Â In addition to providing drug-drug and drug-allergy interaction checks, this criteria requires EHRs to adjust the level of interactions based on user roles (ie: administrator).
Tip:Â Provide an easy-to-use user interface so a user can quickly adjust the interactions. DoseSpot allows the user to turn on and off the drug-drug and drug-allergy interactions as well adjust the severity level display between minor, moderate and major interactions.
Click here for more blog posts at ePrescribing.org on Meaningful Use.


 Follow
Follow





